Research
Laboratory for RNA regulatory mechanisms in muscle development
The Spletter Lab: RNA regulatory mechanisms in muscle development
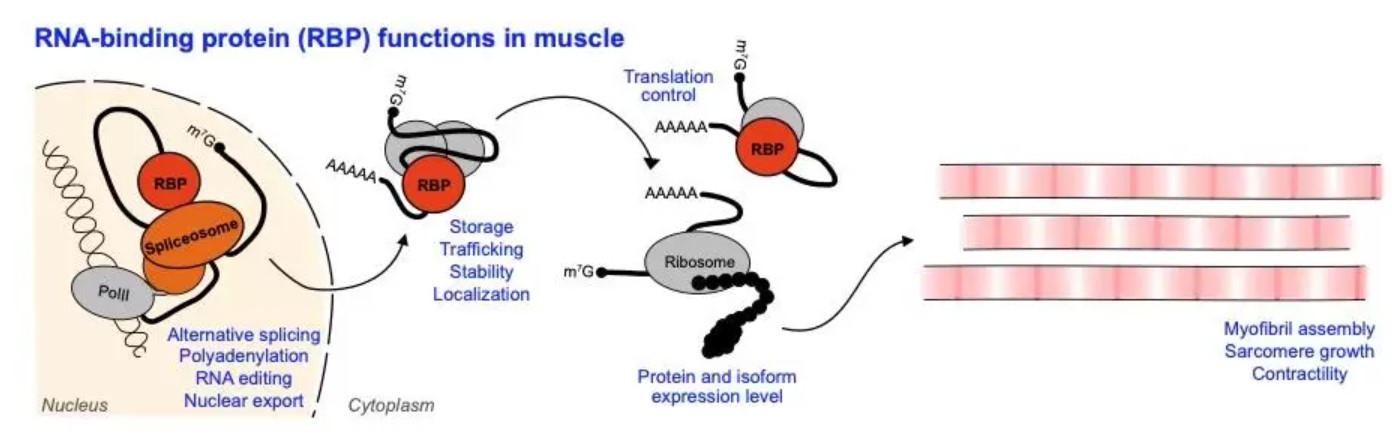
The Spletter lab studies how RNA regulation influences muscle development, as well as which RNA binding proteins (RBPs) are important for myogenesis. After a gene is transcribed, there are multiple regulatory steps that determine if a protein corresponding to that gene will be produced. The pre-RNA needs to be spliced, a process which often happens co-transcriptionally and can lead to the production of distinct splice isoforms through alternative splicing. pre-RNAs need to be tailed, capped and edited to mature them to mRNAs that can be exported from the nucleus. Once in the cytoplasm, mRNAs are trafficked to target destinations, for example to P-granules for storage or to different sub-cellular destinations. RNA binding proteins mediate these various regulatory steps, and ultimately determine the expression level of distinct splice isoforms of structural proteins that are available to assemble sarcomeres. RNA regulation therefore influences the morphology as well as the contractility of different muscle fiber types.
Our Focus
Our mission is to provide a more complete understanding of the regulation and function of RNA processing in muscle development. Along the way, we are identifying which proteins can bind to and regulate RNA, working to gain a deeper understanding of muscle development itself and investigating the function of alternatively spliced isoforms during myogenesis.
Muscle-type specific function of Bruno1 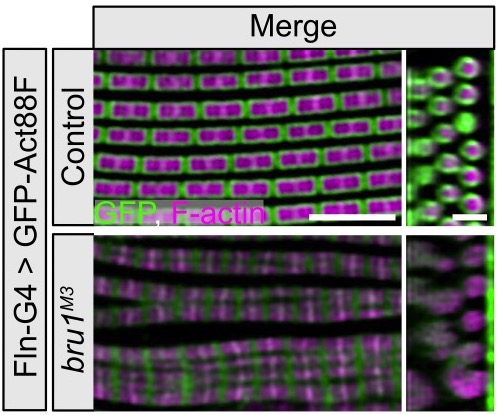
Bruno1 (also called Bru1, Arrest, Aret) is a conserved member of the CELF-family of RNA binding proteins known to be mis-regulated in myotonic dystrophy. Our previous work showed that Bru1 regulates flight muscle specific alternative splicing in Drosophila, and its loss leads to defects in sarcomere growth, hypercontraction and ultimately muscle fiber loss. We continue to investigate the mechanism by which Bru1 regulates splicing in flight muscle, including the developmental mechanisms leading to myofibril phenotypes in Bru1 mutants. We also are evaluating the function of different Bru1 isoforms and working to identify Bru1 binding elements in direct RNA targets. Our findings will have implications for the function of CELF family members in muscle development and disease.
 Research Area #2
Research Area #2
Describe this area of your research and replace/delete all lorem ipsum text.
Ut sem viverra aliquet eget sit amet tellus. Elementum tempus egestas sed sed risus pretium. In hac habitasse platea dictumst vestibulum rhoncus est pellentesque elit.
